Artificial intelligence must be as reliable after upgrade as before
Artificial intelligence allows us to develop better and more efficient products and tools, especially in healthcare. Medical devices of this kind represent a significant added value. However, the upgrades of such computer systems - after they are certified on the market - cause that initial verification and validation to quickly lose evidential value. In the European Vivaldy project (Verification and Validation of Ai-enabLed Systems), VITO worked with Belgian partners such as Barco and icometrix on solutions to cost-effectively re-verify, validate and certify upgrades to AI-based medical devices.
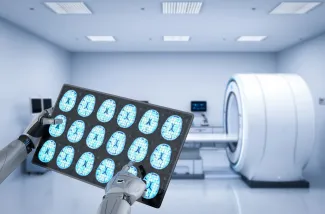
Artificial intelligence is driving many advances, including in healthcare. AI-based medical devices such as e.g. a medical scanner, or a computer programme that can detect a lesion on an MRI scan, can add value in diagnosing conditions and treating patients.
However, it is important that advanced AI-based medical devices can be upgraded regularly after they come to market. These upgrades keep the system functional and allow new improvements to be added. One consequence, however, is that the initial verification and validation of the device quickly loses evidential value, while collecting new medical data each time is time-consuming and expensive.
This applies to all professional healthcare systems where patient safety is paramount. It is crucial that the outcome of those upgrades is as reliable and efficient as the originally certified system.
Re-verify upgrades
Companies like icometrix, Barco and Dutch company Philips face the challenge of cost-effectively re-verifying, validating and certifying upgrades of AI-based medical devices to ensure their intended use, while competing with the development speed of US and Asian companies.
That was precisely the intention of the European Vivaldy project, which is now coming to an end. The results were proven on four industrial use cases, each targeting a specific type of upgrade, ranging from hardware components to AI applications. Through discussions with healthcare certification bodies and partners active in other areas of the safety-critical industry, Vivaldy has disseminated its results and guidelines to a wider audience, contributing to new standards for certification.
This research was funded by the Vivaldy project, PENTA 19021, and financially supported by the Flemish government HBC.2019.274.
Project partners
Philips Healthcare, Barco, icometrix, Unit040, Verum, TU Delft, TNO-ESI, VITO




