Empowering patients: Belgian consortium launches TTP survey with secure health data pods
A Belgian consortium of top scientists and patient representatives launches a pioneering patient survey utilising Personalised Data Pods to secure the patient’s data and to allow them to store and manage their own health information. The survey focuses on patients with TTP (Thrombotic Thrombocytopenic Purpura), a rare blood condition characterised by small blood vessels clotting, leading to symptoms like anemia, neurological symptoms and kidney disease. The method is unique in its kind, fully patient-centric and offers a safe and efficient health data solution.

Gathering insights for improved disease management
The consortium consists of six stakeholders, including the Belgian patient association TTP Community, the research institute VITO (Vlaamse Instelling voor Technologisch Onderzoek), Professors Catherine Lambert (Université Catholique de Louvain), Daan Dierickx and Karen Vanhoorelbeke (Katholieke Universiteit Leuven), alongside pharmaceutical companies Takeda Belgium and Sanofi Belgium. In January 2025, the consortium launched a national patient survey to gather deeper insights in patients with Thrombotic Thrombocytopenic Purpura (TTP).
By collaborating in a consortium, the involved stakeholders will gain insights into the diagnosis, the patient journey, treatment outcomes and disease monitoring of patients. These are critical factors for optimising TTP patient care. Simultaneously, patients participating in the survey remain in full control of their data through a secure personalised data pods system, ensuring transparency, security, and trust in medical research.
Personalised data pods: a new patient-centric approach to data management
To ensure that participating patients remain in full control of their data - the consortium uses a new technology called ‘Personalised Data Pods’ to store personal data. This privacy-preserving technology is now implemented for the first time with the We Are applications and using Athumi pods with underlying SOLID technology. These leverage secure Personalised Data Pods to empower individuals to store and manage their own health information and actively participate in data-sharing decisions.
VITO, through its We Are project (www.we-are-health.be), is actively working on personalised health by involving citizens in the management and sharing of their health data. Central to this initiative is the development and deployment of personalised data pods, designed to be accessible, secure, and ethically sound. The entire TTP survey underwent a thorough review to ensure it met strict standards for security, privacy and ethics. Additionally, VITO provided the necessary tools enabling patients to access their personal data pods and share their information with researchers in a safe and anonymised manner.
The use of Personalised Data Pods aligns with the new vision and standards set by the European Health Data Space regulation. The pods can be considered as tools providing citizens control of their personal data, integrate health data from different sources at one location, and stimulate the secondary use of health data. It is believed that these efforts will ultimately lead to better diagnosis and treatment, improved patient safety, continuity of care and improved healthcare efficiency across Europe.
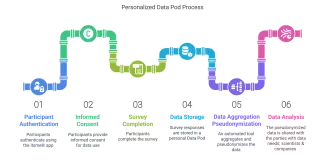
How it works
But how does it work? Participants authenticate themselves via the itsme® application and provide informed consent before completing the survey. Their responses are securely stored within a personal Data Pod. Upon survey completion, an automated tool aggregates and pseudonymises the data for analysis. In return, participants receive a personalised infographic summarising key findings — ensuring an open, transparent exchange of information.
By leading this initiative, the TTP consortium partners aim to establish new benchmarks for ethical, patient-centric health data management, fostering more inclusive and effective medical research in Belgium.
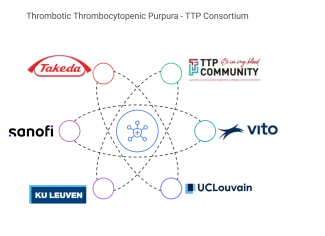
About the TTP consortium
The TTP (Thrombotic Thrombocytopenic Purpura) consortium was created to allow better understanding of the disease by releasing a survey and assuring input from all relevant stakeholders in the field. The consortium consists of the Belgian patient association TTP Community, European research centre VITO, academic partners including Professor Catherine Lambert from Université Catholique de Louvain (UCLouvain), Professor Daan Dierickx and Professor Karen Vanhoorelbeke from Katholieke Universiteit Leuven (KU Leuven) and the pharmaceutical companies Takeda Belgium and Sanofi Belgium.