Synopsis
Urine-based diagnostic tests are under way for non-invasive monitoring of kidney rejection. Ten novel, clinically validated protein biomarkers present in urine will allow for hospital testing within standard platform technology as well as home testing by the patient, using a subset of the biomarker panel. To facilitate market introduction of these diagnostics, currently under development, the partners in this project are seeking to expand the team and prepare for a first round of investment.
Kidney transplantation: a burden for patient & care system
Balancing patient quality of life and immune suppression therapy after transplantation requires adequate & frequent testing for transplant organ rejection. The current kidney transplant follow-up includes recurring kidney biopsies and histopathology: an unpleasant, invasive experience for the patient and a costly, labour-intensive process in the clinical lab.
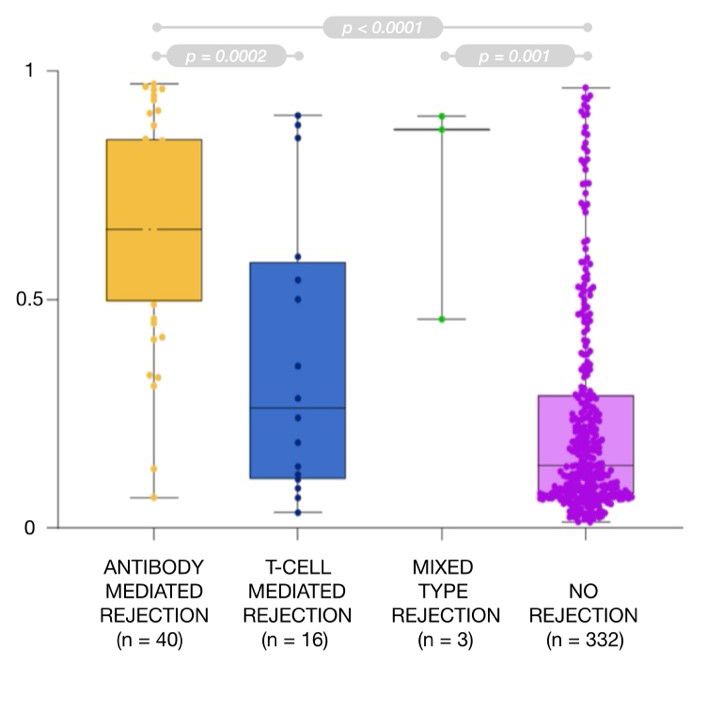
Histopathology is today’s golden standard in kidney rejection, because clear distinction has to be made between several types of rejection. The two most important types are T-cell mediated (TCMR) and antibody-mediated kidney rejection (ABMR), because each type of rejection requires specific therapeutic intervention, and when not treated timely, they are the main causes of graft failure.
In the joint research effort, (BioMargin project), the advanced proteomics platform (Centre for Proteomics) was applied to discover novel kidney rejection protein biomarkers that are excreted in urine.
In a second phase, research by the partners confirmed clinical validation of the discovered biomarkers. A multicentre clinical study, which included 390 subjects, showed that collectively, the biomarker set is capable to diagnose kidney rejection. More important, the data confirmed that the panel also distinguishes unequivocally between antibody-mediated and T-cell mediated (and even mixed) types of kidney rejection. For more information on clinical validation and the ROC charts per biomarker we refer to this publication.
Perspective
This novel kidney rejection protein biomarker panel has the potential to leverage transition to a totally new approach of transplant kidney follow-up. It will enable:
- to move from surgery-based to urinary-sample based testing in both clinic & at home;
- to discriminate between the two major types of kidney rejection;
- to facilitate for more frequent transplant follow-up and most important,
- all the above at a much lower cost for the care system and
- with a lot more convenience for the kidney transplant receiver.
Immediate next steps
The Biomargin partners and diagnostic companies teamed up to develop and validate appropriate diagnostic assays. It is envisioned to create a full solution for convenient home-based and hospital-based testing.
The major deliverable of this collaboration is to create all evidence required to found a startup, which realises the full diagnostic potential of these biomarkers in the diagnostics market worldwide.
To that end we are actively seeking an entrepreneur in residence with experience in the diagnostics market. This person will coordinate the diagnostic assays development plan and market introduction program, will build the startup team; prepare and succesfully conclude a seed investment round in close collaboration with VITO and program partner Tech Transfer Teams.